Prussian blue
| Prussian blue | |
|---|---|
 |
|
|
Iron(II,III) hexacyanoferrate(II,III)
|
|
|
Other names
ferric ferrocyanide, iron(III) ferrocyanide, iron(III) hexacyanoferrate(II), ferric hexacyanoferrate (German: Preußischblau and Berliner Blau, Berlin blue, Parisian blue
|
|
| Identifiers | |
| CAS number | 14038-43-8 |
| PubChem | 167287 (soluble), 2724251, [1] |
| RTECS number | V03AB31 |
| Properties | |
| Molecular formula | K[FeIIIFeII(CN)6] or FeIII4[FeII(CN)6]3.xH2O |
| Molar mass | 859.23 g/mol |
| Appearance | blue microcrystalline powder |
| Solubility in water | insoluble |
| Pharmacology | |
| Routes of administration |
Oral |
| Hazards | |
| MSDS | MSDS prussian blue |
| EU Index | Not listed |
| Flash point | Non-flammable |
| Related compounds | |
| Related compounds | Sodium ferrocyanide Potassium ferrocyanide Potassium ferricyanide |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
| Prussian Blue | ||
|---|---|---|
|
— Color coordinates — |
||
| Hex triplet | #003153 | |
| RGBB | (r, g, b) | (0, 49, 83) |
| HSV | (h, s, v) | (205°, 100%, 43%) |
| Source | [Unsourced] | |
| B: Normalized to [0–255] (byte) |
||
Prussian blue is a dark blue pigment with the idealized formula Fe7(CN)18⋅14H2O.[1] Another name for the color Prussian blue is Berlin blue or, in painting, Parisian blue. Turnbull's blue is the same substance but is made from different reagents.
Prussian blue is one of the first synthetic pigments. The pigment is famously complex, owing to the presence of variable amounts of other ions and the sensitive dependence of its appearance to the size of the particles. The pigment is used in paints, and it is the traditional "blue" in blueprints. It has been used as an antidote for certain kinds of heavy metal poisoning.
Contents |
History
Prussian blue was probably synthesized for the first time by the paint maker Diesbach in Berlin around the year 1706.[2] Most historical sources do not mention a first name of Diesbach. Only Berger refers to him as Johann Jacob Diesbach.[3] It was named "Preußisch blau" and "Berlinisch Blau" in 1709 by its first trader.[4] The pigment was an important topic in the letters exchanged between Johann Leonhard Frisch and the president of the Royal Academy of Sciences, Gottfried Wilhelm Leibniz, between 1708 and 1716.[4] It is first mentioned in a letter written by Frisch to Leibniz, from March 31, 1708. Not later than 1708, Frisch began to promote and sell the pigment across Europe. By August 1709, the pigment had been termed "Preussisch blau"; by November 1709, the German name "Berlinisch Blau" had been used for the first time by Frisch. Frisch himself is the author of the first known publication of Prussian blue in the paper Notitia Coerulei Berolinensis nuper inventi in 1710, as can be deduced from his letters. Diesbach had been working for Frisch since about 1701.
In 1731, Georg Ernst Stahl published a story dealing with the incidence of the first synthesis of Prussian blue.[5] The story involves not only Diesbach but also Johann Konrad Dippel. Diesbach was attempting to create a red lake pigment from cochineal but obtained the blue instead as a result of the contaminated potash he was using. He borrowed the potash from Dippel, who had used it to produce his "animal oil". No other known historical source mentions Dippel in this context. It is therefore difficult to judge the reliability of this story today. In 1724, the recipe was finally published by Woodward.[6]
To date, the "Entombment of Christ", dated 1709 by Pieter van der Werff (Picture Gallery, Sanssouci, Potsdam) is the oldest known painting where Prussian blue was used. Around 1710, painters at the Prussian court were already using the pigment. At around the same time, Prussian blue arrived in Paris, where Antoine Watteau and later his successors Nicolas Lancret and Jean-Baptiste Pater used it in their paintings.[7]

This Prussian blue pigment is significant since it was the first stable and relatively lightfast blue pigment to be widely used following the loss of knowledge regarding the synthesis of Egyptian Blue. European painters had previously used a number of pigments such as indigo dye, smalt, and Tyrian purple, which tend to fade, and the extremely expensive ultramarine made from lapis lazuli. Japanese painters and woodblock print artists likewise did not have access to a long-lasting blue pigment until they began to import Prussian blue from Europe. Cobalt blue has been used extensively by Chinese artists in blue and white porcelains for centuries, and was introduced to Europe in the 18th century.
In 1752 the French chemist Pierre J. Macquer made the important step of showing the Prussian blue could be reduced to a salt of iron, and a new acid, which could be used to reconstitute the dye. The new acid, hydrogen cyanide, first isolated from Prussian blue in pure form and characterized about 1783 by the Swedish chemist Carl Wilhelm Scheele, was eventually given the name Blausäure (literally "Blue acid") because of its derivation from Prussian blue, and in English became known popularly as Prussic acid. Prussian blue would also give the name to the cyanide family of compounds, which are named from the Greek word for "blue," because they were first isolated from Prussian blue.
Production
Prussian blue has a "soluble" form, K[FeIIIFeII(CN)6], and an "insoluble" form, FeIII4[FeII(CN)6]3.xH2O.
- Soluble Prussian blue can be made from potassium ferrocyanide and iron(III):
- K+ + Fe3+ + [FeII(CN)6]4- → KFeIII[FeII(CN)6]
The similar reaction of potassium ferricyanide and iron(II) results in the same colloidal solution, because [FeIII(CN)6]3- is converted into ferrocyanide, as the following equilibrium lies on the right-hand side:
- Insoluble Prussian blue is produced if in the reactions above an excess of Fe3+ or Fe2+, respectively, is added. In the first case:
- 4Fe3+ + 3[FeII(CN)6]4- → FeIII[FeIIIFeII(CN)6]3 [8]
Soluble Prussian blue for use in inks is prepared by adding a solution containing iron(III) chloride to a solution of potassium ferrocyanide. During the course of the addition the solution thickens visibly and the color changes immediately to the characteristic blue of Prussian blue.
Turnbull's blue
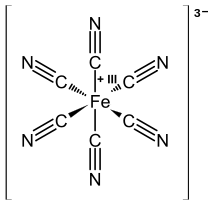
Whereas "Prussian blue" (PB) can be obtained by two different methods, either by addition of a Fe(III) salt to a solution of ferrocyanide or by addition of a Fe(II) salt to a solution of ferricyanide, in former times the methods were thought to produce two different products.
The product of Fe(II) and [Fe(CN)6]3- was traditionally named "Turnbull's Blue" (TB). It has been shown, however, by means of X-ray diffraction and electron diffraction methods, that the structures of PB and TB are identical.[9][10] The differences in the colors for TB and PB reflect subtle differences in the method of precipitation, which strongly affects particle size and impurity content.
Properties
Prussian blue is a microcrystalline blue powder. It is insoluble, but the crystallites tend to form a colloid. Such colloids can pass through fine filters[11]. Despite being one of the oldest known synthetic compounds, the composition of Prussian blue remained uncertain for many years. The precise identification of Prussian blue was complicated by three factors:
- Prussian blue is extremely insoluble but also tends to form colloids;
- Traditional syntheses tend to afford impure compositions;
- Even pure Prussian blue is structurally complex, defying routine crystallographic analysis.
Crystal structure
The chemical formula of insoluble Prussian blue is Fe7(CN)18.xH2O, where x = 14-16. The structure was determined by using IR spectroscopy, Moessbauer spectroscopy, X-ray crystallography, and neutron crystallography. Since X-ray diffraction cannot distinguish carbon from nitrogen, the location of these lighter elements is deduced by spectroscopic means as well as by observing the distances from the iron atom centers.
PB has a cubic lattice structure. Soluble PB crystals contain interstitial K+ ions; insoluble PB has interstitial water instead.
In ideal insoluble PB crystals, the cubic framework is built from Fe(II)-C-N-Fe(III) sequences, with Fe(II)-carbon distances of 1.92 Å (0.192 nanometers) and Fe(III)-nitrogen distances of 2.03 Å (0.203 nanometers). One-fourth of the sites of Fe(CN)6 subunits are vacant (empty), leaving three such groups. The empty nitrogen sites are filled with water molecules instead, which are coordinated to Fe(III).
The Fe(II) centers, which are low spin, are surrounded by six carbon ligands in an octahedral configuration. The Fe(III) centers, which are high spin, are octahedrally surrounded on average by 4.5 nitrogen atoms and 1.5 oxygen atoms (the oxygen from the six coordinated water molecules). Additional eight (interstitial) water molecules are present in the unit cell, either as isolated molecules or hydrogen bonded to the coordinated water.
The composition is notoriously variable due to the presence of lattice defects, allowing it to be hydrated to various degrees as water molecules are incorporated into the structure to occupy cation vacancies. The variability of Prussian blue's composition is attributable to its low solubility, which leads to its rapid precipitation without the time to achieve full equilibrium between solid and liquid. [1] [12]
Color
Prussian blue is strongly colored and tends towards black and dark purple when mixed into oil paints. The exact hue depends on the method of preparation, which dictates the particle size. The intense blue color of Prussian blue is associated with the energy of the transfer of electrons from Fe(II) to Fe(III). Many such mixed-valence compounds absorb certain wavelengths of visible light resulting from intervalence charge transfer. In this case, orange-red light around 680 nanometers in wavelength is absorbed, and the transmitted light appears blue as a result.
PB is electrochromic — changing from blue to colorless upon reduction. This change is caused by reduction of the Fe(III) to Fe(II) eliminating the intervalence charge transfer that causes Prussian blue's color.
Other properties
- Despite the presence of the cyanide ion, Prussian blue is not especially toxic because the cyanide groups are tightly bound to Fe. Other cyanometalates are similarly stable with low toxicity. Treatment with acids, however, can liberate the extremely toxic hydrogen cyanide.
Uses
Prussian blue in medicine
Prussian blue's ability to incorporate monocations makes it useful as a sequestering agent for certain heavy metal poisons. Pharmaceutical-grade Prussian blue in particular is used for patients who have ingested thallium or radioactive caesium. According to the International Atomic Energy Agency, an adult male can eat at least 10 grams of Prussian blue per day without serious harm. The U.S. Food and Drug Administration (FDA) has determined that the "500 mg Prussian blue capsules, when manufactured under the conditions of an approved New Drug Application (NDA), can be found safe and effective therapy" in certain poisoning cases.[13] Radiogardase (Prussian blue insoluble capsules [14]) is a commercial product for the removal of caesium-137 from the bloodstream.[15]
As a laboratory histopathology stain for iron

Prussian blue is a common histopathology stain used by pathologists to detect the presence of iron in biopsy specimens, such as in bone marrow samples. The original stain formula, known historically (1867) as "Perls' Prussian blue" after its inventor, German pathologist Max Perls (1843-1881), used separate solutions of potassium ferrocyanide and acid to stain tissue (these are now used combined, just before staining). Iron deposits in tissue then form the purple Prussian blue dye in place, and are visualized as blue or purple deposits.[16] The formula is also known as Perls Prussian blue and (incorrectly) as Perl's Prussian blue.
As a pigment
Prussian blue is the coloring agent used in engineer's blue and the pigment formed on cyanotypes - giving them their common name blueprints. Certain crayons were once colored with Prussian blue (later relabeled Midnight Blue). It is also a popular pigment in paints. Similarly, Prussian blue is the basis for laundry bluing.
By machinists and toolmakers
Prussian blue in oil paint is the traditional material used for spotting metal surfaces such as surface plates and bearings for hand scraping. A thin layer of non-drying paste is applied to a reference surface and transfers to the high spots of the workpiece. The toolmaker then scrapes, stones, or otherwise removes the high spots. Prussian blue is preferable because it will not abrade the extremely precise reference surfaces as many ground pigments may.
Analytical chemistry
Prussian blue is formed in the Prussian blue assay for total phenols. Samples and phenolic standards are given acidic ferric chloride and ferricyanide which is reduced to ferrocyanide by the phenols. The ferric chloride and ferrocyanide react to form Prussian blue. Comparing the absorbance at 700 nm of the samples to the standards allows for the determination of total phenols.[17]
See also
- Potassium ferrocyanide
- Potassium ferricyanide
- Methyl blue
- Methylene blue
- New methylene blue
- Egyptian Blue
- Han Purple
- Gentian violet
- Fluorescein
External links
- The FDA's page on prussian blue
- The CDC's page on prussian blue
- National Pollutant Inventory - Cyanide compounds fact sheet
- Heyltex Corporation distributors of Radiogardase (Prussian blue insoluble capsules)
- Sarah Lowengard, "Prussian Blue" in The Creation of Color in Eighteenth Century Europe Columbia University Press, 2006
References
- ↑ 1.0 1.1 F. Herren, P. Fischer, A. Ludi, W. Haelg "Neutron diffraction study of Prussian Blue, Fe4[Fe(CN)63.xH2O. Location of water molecules and long-range magnetic order"] Inorg. Chem., 1980, 19 (4), pp 956–959.doi:10.1021/ic50206a032
- ↑ Jens Bartoll. "The early use of prussian blue in paintings" (PDF). 9th International Conference on NDT of Art, Jerusalem Israel, 25-30 May 2008. http://www.ndt.net/article/art2008/papers/029bartoll.pdf. Retrieved 2010-01-22.
- ↑ J. E. Berger: Kerrn aller Fridrichs=Städtschen Begebenheiten Manuskript, Berlin, ca.1730 (Berlin, Staatsbibliothek zu Berlin – Preußischer Kulturbesitz, Handschriftenabteilung, Ms. Boruss. quart. 124)
- ↑ 4.0 4.1 J. L. Frisch: Briefwechsel mit Gottfried Wilhelm Leibniz L. H. Fischer (ed.), Berlin, Stankiewicz Buchdruck, 1896, reprint Hildesheim/New York: Georg Olms Verlag, 1976
- ↑ G. E. Stahl: Experimenta, Observationes, Animadversiones CCC Numero, Chymicae et Physicae, Berlin, 1731
- ↑ J. Woodward: Praeparatio coerulei Prussiaci es Germanica missa in: Philosophical Transactions, London, 33, 1726
- ↑ J.Bartoll, B. Jackisch, M. Most, E. Wenders de Calisse, C. M. Vogtherr: Early Prussian Blue. Blue and green pigments in the paintings by Watteau, Lancret and Pater in the collection of Frederick II of Prussia In: TECHNE 25, 2007, S. 39-46
- ↑ Egon Wiberg,Nils Wiberg,Arnold Frederick Holleman: Inorganic chemistry, p.1444. Academic Press, 2001; Google books
- ↑ Toru Ozeki, Koichi Matsumoto, Seiichiro Hikime: Photoacoustic spectra of prussian blue and photochemical reaction of ferric ferricyanide Anal. Chem., 1984, 56 (14), pp. 2819–2822. doi:10.1021/ac00278a041
- ↑ Izatt, et al. Calorimetric study of Prussian blue and Turnbull's blue formation Inorg. Chem., 1970, 9 (9), pp. 2019–2021. doi:10.1021/ic50091a012
- ↑ *Dunbar, K. R. and Heintz, R. A., "Chemistry of Transition Metal Cyanide Compounds: Modern Perspectives", Progress in Inorganic Chemistry, 1997, 45, 283-391.
- ↑ C. A. Lundgren, Royce W. Murray: Observations on the composition of Prussian blue films and their electrochemistry Inorg. Chem., 1988, 27 (5), pp 933–939; doi:10.1021/ic00278a036
- ↑ "Questions and Answers on Prussian Blue". http://www.fda.gov/Drugs/EmergencyPreparedness/BioterrorismandDrugPreparedness/ucm130337.htm. Retrieved 2009-06-06.
- ↑ Radiogardase: Package insert with formula
- ↑ Heyltex Corporation - Toxicology
- ↑ Formula for Perls' Prussian blue stain. Accessed April 2, 2009.
- ↑ Tannin ChemistryPDF (1.41 MB)Accessed December 19, 2009
| Shades of blue | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Air Force blue | Alice blue | Azure | Baby blue | Bleu de France | Blue | Bondi blue | Brandeis blue | Cambridge Blue | Carolina blue |
| Ceil | Cerulean | Cobalt blue | Columbia blue | Cornflower blue | Cyan | Dark blue | Deep sky blue | Denim | Dodger blue |
| Duke blue | Egyptian blue | Electric blue | Eton blue | Federal Blue | Glaucous | Han blue | Iceberg | Indigo | International Klein Blue |
| Iris | Light blue | Majorelle Blue | Maya blue | Midnight blue | Navy blue | Non-photo blue | Palatinate blue | Periwinkle | Persian blue |
| Powder blue | Prussian blue | Royal blue | Sapphire | Sky blue | Steel blue | Teal | Tiffany Blue | Tufts Blue | UCLA Blue |
| Ultramarine | Yale Blue | ||||||||
| The samples shown above are only indicative. | |||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
![\mathrm{Fe^{2+} + [Fe(CN)_6]^{3-} \ \rightleftharpoons \ Fe^{3+} + [Fe(CN)_6]^{4-}}](/2010-wikipedia_en_wp1-0.8_orig_2010-12/I/d5990cc9a5d27f3351e6f29c5f1d2969.png)